Unit 10:
Electron Configurations
Unit Overview:
In
the last unit, you continued your examination of the development of the model
of the atom, focusing on deepening your understanding of the structural
components of the atom. You clarified
the nuclear model by considering the planetary model.
|
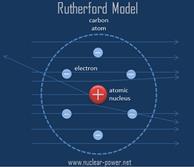
The
Nuclear Model:
|
What it’s based on: ● Rutherford’s Gold Foil Experiment ● The discovery of the nucleus |
Unanswered Questions of this model: 1. How do we describe the
nucleus of the atom? 2. Why do the electrons stay
outside of the nucleus of an atom? |
|
The
Planetary Model:
|
What it’s based on: ● An extension of
Rutherford’s Gold Foil Experiment → protons. ● Chadwick’s
Beryllium Experiment → neutrons. ● Bohr’s Hydrogen
Model → energy levels. |
How it answers the Questions of the Nuclear
Model: 1. It explains that the
nucleus is made up of protons and neutrons. 2. It explains that electrons
are found within energy levels outside of the nucleus. |
In
this unit, you will further deepen your understanding of the electrons within
the energy levels of an atom by considering the development of the quantum
model of the atom.
What is the flaw of the Planetary
Model of the Atom?
Although
the planetary model more accurately represents the nucleus of the atom by
indicating that the nucleus is comprised of both protons and neutrons, its
representation of the electrons is overly-simplistic. Recall that Niels Bohr examined the Hydrogen
atom, creating a mathematical model to explain its emission spectrum. This model accurately describes the movement
of hydrogen’s single electron between specific energy levels in the atom.
However,
as scientists attempted to extend this mathematical model to larger atoms, they
noticed that it did not perfectly predict the emission spectra of larger
atoms. Although it generally predicted
its spectral lines, it did not accurately predict the wavelength of each line. This inability to perfectly predict the exact
spectra motivated scientists implied that the planetary model’s explanation
that the electron travels in a fixed path around the nucleus of the atom. This inaccuracy motivated scientists to
continue to explore the location of the electron within the electron cloud,
once again changing the model of the atom over time.
Three
important contributions helped us to understand the flaws of the planetary
model:
1. Dual Wave-Particle Nature of the Electron
In 1924, Louis de Broglie
hypothesized that because of the extremely small size of the
Electron it is not sufficient to conceive of them
simply as particles. Instead, he
suggested that electrons also have wave properties
associated with them. This
hypothesis helps to explain the inaccuracy of the planetary
model by offering a reason for it - the electron is not simply a particle.
In
order to hear a verbal description about this dual wave-particle nature, watch
the following video: Video
2. Wave Equation
In 1926, Erwin Schrodinger used mathematical equations to describe the probability of finding an electron in a certain position outside of the nucleus. These equations provide a three-dimensional description of the electron cloud. This explanation applied the wave nature of the electron to the atomic model to understand areas of probability within energy levels where it is likely to find an electron. Video
3. Uncertainty Principle
In 1927, Werner Heisenberg explained that it was
impossible to know the exact position and momentum of the electron. In other words, because of the dual
wave-particle nature of the electron, it is impossible to know its exact location
within the atom at a given moment. This explanation helps to understand that
the electron is not on a fixed path like the planetary model indicates.
In
order to hear a verbal description and watch a laser experiment about
Heisenberg’s Uncertainty Principle, watch the following video: Video
What is the Quantum Mechanical Model
of the Atom?
Based
on Schrodinger’s wave equation, scientists changed the model of the atom -
removing the electron from the fixed orbit of the planetary model, and instead
describing the electron within the most probable areas of the electron cloud
where it can be found. This most current model of the atom is referred to as
the quantum mechanical model of the atom.
This model is described by quantum numbers.
The quantum numbers are a set of 4 numbers
that describe the electron and its location in the electron cloud of the
atom. In order to understand these
numbers, we are going to define them and use an analogy to understand
them. In this analogy, we will compare
the quantum numbers to an apartment building.
The electron cloud is the general region
outside of the nucleus in which all of the electrons of an atom are found. In our
analogy, an apartment building is similar to the electron cloud. The four
quantum numbers further divide the electron cloud to better describe the
location of the electron.
1.
Principal Quantum
Number - describes the energy
level
●
Symbol: n
●
Possible values: n
= 1, 2, 3, 4, 5, 6, 7 {positive integer values}
●
Special notes:
○
The higher the
value of n, the higher the energy level.
○
The higher the
energy level, the further away from the nucleus.
●
Analogy: The principal quantum number divides the
electron cloud into energy levels, just like an apartment building is divided
into floors.
2. Angular Momentum [Azimuthal] Quantum Number - describes the sublevel within the energy level
●
Symbol: l
●
Possible values: l = 0
- (n-1) {a range of values}
●
Special notes: also
describes the general shape and number of the orbitals within that sublevel
○
When l = 0,
there is 1 orbital in the sublevel, it is referred to as an s-orbital, and has
a spherical shape
○
When l = 1,
there are 3 orbitals in the sublevel, they referred to as a p-orbital, and have
a 2-lobe shape
○
When l = 2,
there are 5 orbitals in the sublevel, they referred to as a d-orbital, and have
a 4-lobe shape
○
When l = 3,
there are 7 orbitals in the sublevel, they referred to as a f-orbital, and have
a 8-lobe shape
●
Analogy: The angular momentum quantum number
divides the energy levels into sublevels, just like the floors of an apartment
building are divided into apartments.
3. Magnetic Quantum Number - describes the orbital within the sublevel
●
Symbol: ml =
●
Possible
values: ml = -l -
0 - +l {a
range of values}
●
Special notes:
○
The orbital is
the specific area within the electron cloud in which it is most likely to find
the electron.
○
Orbitals within
a sub-level have equal energy.
●
Analogy: The magnetic quantum number divides
the sublevel into orbitals, just like an apartment is divided into separate
rooms.
4. Spin Quantum Number - describes the spin of the electron within
the orbital
●
Symbol: ms
●
Possible
values: ms = +½ or -½
●
Special notes:
○
There are only
2 possible values of the spin quantum number
■
There are only
2 different directions in which the electron can spin.
■
These 2
directions are represented as an up and a down arrow.
○
There is a
maximum of 2 electrons in any one orbital.
○
If 2 electrons
occupy the same orbital, they must have opposite spins.
●
Analogy: The spin quantum number describes the
electron(s) within the orbital, just like people can be in the rooms of an
apartment; the maximum occupancy of any room is 2.
Visualizing our
Analogy:
Electron-Land Apartments:
|
l = 0 n= 4 ⇅ ml = 0 |
l = 1 ⇅ ml = -1 |
⇅ ml = 0 |
⇅ ml = +1 |
l = 2 ⇅ ml = -2 |
⇅ ml = -1 |
⇅ ml = 0 |
⇅ ml = +1 |
⇅ ml = +2 |
l=3 ⇅ ml = -3 |
⇅ ml = -2 |
⇅ ml = -1 |
⇅ ml = 0 |
⇅ ml = +1 |
⇅ ml = +2 |
⇅ ml = +3 |
||||||||
|
l = 0 n = 3⇅ ml = 0 |
l = 1 ⇅ ml = -1 |
⇅ ml = 0 |
⇅ ml = +1 |
l = 2 ⇅ ml = -2 |
⇅ ml = -1 |
⇅ ml = 0 |
⇅ ml = +1 |
⇅ ml = +2 |
|||||||||||||||
|
l = 0 n = 2 ⇅ ml = 0 |
l = 1 ⇅ ml = -1 |
⇅ ml = 0 |
⇅ ml = +1 |
||||||||||||||||||||
|
l = 0 n = 1
⇅ ml = 0 |
|||||||||||||||||||||||
In
order to hear a verbal description and see more visual aids to help you to
understand the quantum mechanical model of the atom, watch the following video: Video
Because
of the abstract nature of this model of the atom, you may find it helpful to
watch a second video that represents the orbitals within an atom: Video
Practice: Complete this online quiz about the quantum mechanical
model of the atom.
What are electron configurations?
Although
the planetary model is not accurate because we know that the electrons are not
traveling on fixed paths, it is a simpler representation of the atom to draw -
a drawing of the quantum mechanical model is too complex to draw because it is
difficult to draw the electrons in the numerous orbitals inside that exist in
the concentric energy levels. Therefore,
the quantum model is more often represented as electron configurations.
The electron configuration is a simplified
representation of the filling of the electrons in the electron cloud of an
atom.
The
writing of electron configurations is guided by several principals:
1. The Aufbau Principle states that electrons fill sublevels in order of
increasing energy. It is often referred
to as the “building up principle” because it emphasizes that electrons fill
lower energy levels before they begin filling higher energy levels. So, in general, the 1st energy level fills
before the second, which fills before the third, etc. And, within energy
levels, the s sublevel is filled before the p sublevel, which fills before the
d sublevel, which fills before the f sublevel.
However, there are some exceptions to his principle when filling the d
and f sublevels because of their complexity. Fortunately, the periodic table reminds us
when these exceptions happen.
2.
Hund’s Rule states that equal-energy
orbitals fill one at a time. In other
words, each orbital will hold 1 electron before any orbitals hold a second
electron.
3.
Pauli’s Exclusion Principle states that no two electrons
within any one atom can have the exact same set of 4 quantum numbers. Therefore, when a second electron goes into
an orbital, it must have the opposite spin.
Consider
the element oxygen in the following examples.
Oxygen has 8 electrons in its electron cloud. There are two primary ways
in which an electron configuration can be written:
- Orbital Notation -
this notation represents the electrons as arrows in simplified diagrams of
the orbitals
Example:
● Description: Each box
represents an orbital. Each arrow
represents an electron. Notice:
○ There is a maximum of 2
electrons in any 1 orbital, 2 arrows in any one box.
○ If there are 2 electrons,
they have opposite spins, an up and a down arrow in any one box.
○ Equal energy orbitals are
represented as boxes that are touching.
2.
Electron Configuration
Notation
- this notation represents the number of electrons in each sublevel
● Example: 1s22s22p4
● Notes: The superscript
represents the total number of electrons that are in the designated sublevel.
In order to hear verbal descriptions and see more visual aids to help you to understand electron configurations of the atom, watch the following video:
Practice: Complete this online practice about electron
configurations, also referring to any of the related pool of videos to help you
better understand this complex concept.
.
ChemLab: Electron Configuration
Overview:
The
quantum mechanical model of the atom provides detailed descriptions of the
electron cloud. In this lab, you will
create the electron configuration of elements by filling electron orbitals. You
will notice patterns in how the electrons fill the electron cloud.
Directions:
1. Download the Student
Exploration and Vocabulary sheets
for Electron Configuration.
2. Familiarize yourself with the
words on the vocabulary sheet.
3. Log-in to your Explore Learning account.
4. Click on “Electron
Configuration” and launch the gizmo.
5. Answer the Prior Knowledge
Question.
6. Practice using the Gizmo,
using the Gizmo warm-up instructions.
7.
After you are comfortable using the Gizmo, begin the
activity. Use the lab sheet as a guide to complete the 2 activities:
a. Activity A: Smaller Atoms
b. Activity B: Larger Atoms
BrainPOP Activity
Overview:
This
unit has been very abstract - trying to visualize and represent the model of
the atom at such a small scale - a scale so small that we cannot actually see
these orbitals that we are describing.
These representations are also very complex, making it difficult to even
draw it. So, it is important to “pull
ourselves back out” and remember why it is important to understand atoms at
this scale. In this activity, you will explore some applications of our atomic
understanding by considering nanotechnology.
Directions:
1. Go to BrainPOP and watch the video on Nanotechnology.
2. To check your understanding,
complete the quiz.
3.
IMPORTANT: This is considered an off-line activity, be
sure to keep track of the time that you spend on BrainPOP.